Global Allergy Point-of-Care Testing Market to Reach USD 4.3 Billion by 2036 on Rapid Diagnostics Adoption
New research forecasts sustained growth in decentralized allergy testing driven by rising prevalence, technological innovation, and primary care demand.
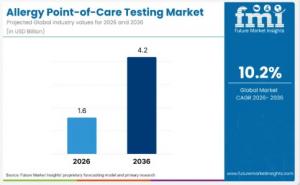
NEWARK, DE, UNITED STATES, February 6, 2026 /EINPresswire.com/ -- The global Allergy Point-of-Care Testing Market is projected to expand significantly through the forecast period, driven by increasing incidence of allergic conditions, demand for rapid diagnostic results, and broader adoption of decentralized healthcare solutions. According to recent market analysis, the sector is anticipated to reach an estimated USD 4.3 billion by 2036, representing a decade of steady growth in clinical utility and service deployment.
Allergy point-of-care testing enables clinicians to identify allergic sensitization and immune responses at or near the patient location—often yielding actionable results within minutes, bypassing traditional laboratory turnaround times. This immediacy supports improved clinical decision-making in primary care, pediatric clinics, emergency departments, and pharmacy-led testing programs worldwide.
WHO: Leading Diagnostics Manufacturers and Healthcare Providers
Key players in the allergy point-of-care testing ecosystem include major diagnostics firms such as Thermo Fisher Scientific, Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, and bioMérieux, among others. These companies are investing in rapid specific-IgE kits, analyzers, and consumables that cater to decentralized testing workflows and evolving clinician needs.
WHAT: Market Drivers and Formats
The allergy point-of-care testing market comprises a range of formats including rapid specific-IgE kits, skin-prick testing kits, rapid anaphylaxis kits, consumables, and associated analyzers. Rapid specific-IgE kits are currently leading in adoption due to their ease of use and consistent performance across diverse clinical settings.
Demand drivers include:
• Urgent clinical decision needs in decentralized care settings
• Rising prevalence of allergic diseases globally
• Preference for same-visit diagnostic clarity to support patient management pathways
• Continued technological improvements in assay design and portability
WHEN & WHERE: Forecast Period and Global Reach
The forecast period extends to 2036, with growth observed across all major regions including North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America and Europe currently anchor substantial market share due to established healthcare infrastructure and strong primary care utilization. Asia Pacific is emerging as a high-growth region, driven by increased healthcare access and expanding outpatient diagnostic services.
WHY: Clinical and Economic Significance
Point-of-care allergy tests provide clear clinical value by delivering timely results that can influence immediate care decisions: whether to treat symptomatically, pursue specialist referral, or initiate allergen avoidance strategies. These tests reduce delays inherent to centralized laboratory workflows, lowering overall patient burden and facilitating better resource allocation across healthcare systems.
Healthcare stakeholders are increasingly prioritizing cost effectiveness, reliability, ease of use, and compatibility with electronic health records when selecting point-of-care diagnostics. Integration with digital health ecosystems supports longitudinal patient tracking and quality metrics, further embedding point-of-care testing within routine clinical practice.
HOW: Technology and Adoption Trends
Market expansion is bolstered by technological innovation such as enhanced assay sensitivity, simplified sample handling, and reduction in operator dependencies. Point-of-care formats that deliver rapid turnaround and reproducible results are particularly favored in primary care triage and urgent care environments.
Training for consistent interpretation and quality control, as well as regional procurement efforts, are shaping adoption patterns. As point-of-care testing technologies mature, procurement teams and providers alike are emphasizing solutions that align with existing clinical workflows and regulatory compliance frameworks.
MARKET CONTEXT AND INDUSTRY RELEVANCE
The broader allergy diagnostics sector remains robust, with complementary markets such as comprehensive laboratory diagnostics and specialized skin testing expanding in parallel. Allergic conditions—ranging from respiratory allergies to food sensitivities—continue to increase in prevalence due to environmental factors, urbanization, and heightened health awareness among populations worldwide.
Allergy point-of-care testing plays a strategic role within this ecosystem by enabling earlier intervention, supporting preventive care models, and enhancing patient engagement through immediate feedback loops. Its relevance extends to health systems seeking to improve clinical outcomes while managing costs and resource utilization.
Request for Sample Report | Customize Report |purchase Full Report – https://www.futuremarketinsights.com/reports/sample/rep-gb-31857
Explore More Related Studies Published by FMI Research:
Surgical Navigation System Market https://www.futuremarketinsights.com/reports/surgical-navigation-system-market
Systemic Lupus Erythematosus SLE Drugs Market https://www.futuremarketinsights.com/reports/systemic-lupus-erythematous-sle-drugs-market
Virology Market https://www.futuremarketinsights.com/reports/virology-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Why Choose FMI: https://www.futuremarketinsights.com/why-fmi
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
rahul.singh@futuremarketinsights.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.